PharaGlow - tracking locomotion and feeding behavior of moving worms
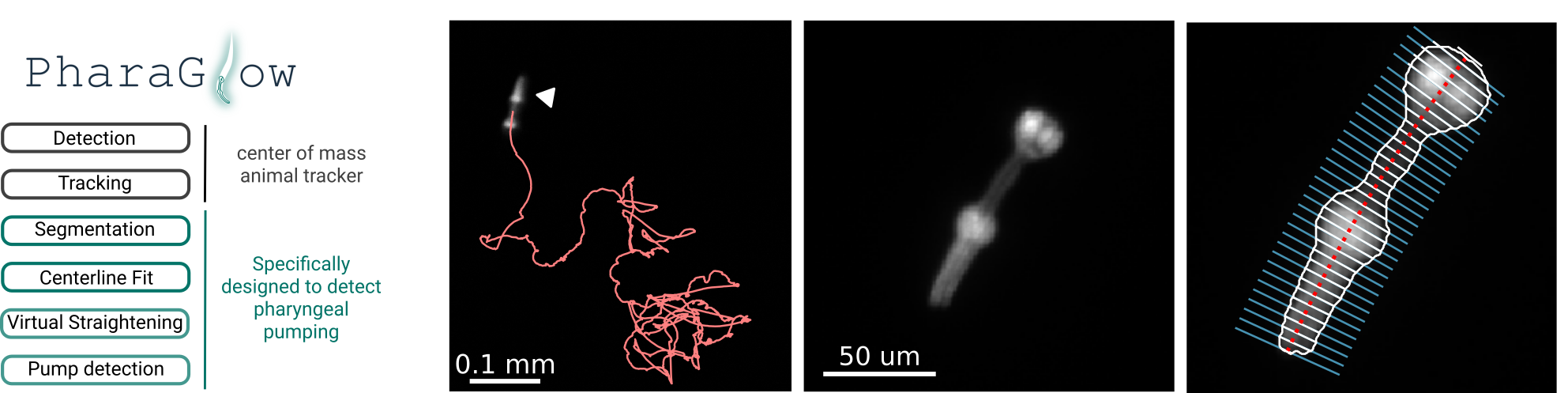
PharaGlow is a python package for tracking and analyzing C. elegans motion and feeding behavior from videos. The package can be used to simply track labelled pharynxes (or whole animals from brightfield) as a simple center of mass tracker, but it also has a pipeline to extract pharyngeal pumping and features of the pharynx.
Installation
Install Anaconda You need to have Anaconda (https://www.anaconda.com/products/individual) and Python >=3.7. We recommend using Anaconda to install Python.
Clone PharaGlow repository from Github in your local directory
Copy the repository link from Github in https://github.com/scholz-lab/PharaGlow/ (in Branch Master > Code > HTTPS (OR SSH))
In the terminal (Linux)/Anaconda Command Prompt (Windows), navigate to the directory where to clone PharaGlow and write the git clone command:
git clone https://github.com/scholz-lab/PharaGlow.git
Note that you can also download PharaGlow from our Github repository (Branch Master > Code > Download ZIP) to have the current copy of PharaGlow.
Create and activate the required anaconda environment In the terminal (Linux)/Anaconda Command Prompt (Windows), naviguate to your newly cloned PharaGlow directory and run:
conda env create --file environmentPumping.yml
You can now use this environment by running:
conda activate pumping
Install PharaGlow
Last step, (don’t forget to activate the pumping environment) run:
python setup.py install --user
(optional) Create a dedicated environment kernel
conda activate myenv
python -m ipykernel install --user --name myenv --display-name "Python (myenv)"
And remove notebook output before committing
conda install -c conda-forge nbstripout
Overview
1. Step - Basic object detection This step creates a “_features.json” file which contains a table of objects (worms) detected in each frame. It creates also a stack of images that contain a cropped area around each worm.
2. Step - Linking objects into trajectories This step results in individual files “_trajectory.json” and _images.tiff for each tracked animal.
3. Step - Analyzing the details of object shapes This step is doing the heavy lifting: It extracts centerlines, widths, contours and other object descriptors from the objects. It results in individual files “_result.json” for each tracked animal.
All subsequent analyses steps add ‘columns’ to the dataframe, and thus features is a subset of trajectories is a subset of results.
Quick Start
Run PharaGlow on a demo dataset
We provide a demo data set with 1000 frames of 1x magnification (30 fps, 2.34 um per pixel) showing C. elegans expressing myo-2::mCherry. https://osf.io/fy4ed/. You can also find the expected outputs in the data repository at OSF.
Before analyzing your data, we recommend to check your installation and familiarize yourself with the code by running the jupyter notebook “testing/PharaGlowMain_testdata.ipynb” on this dataset using the provided parameter file “AnalysisParameters_1x.json”
This notebook runs the pharaglow image analysis pipeline. It comprises three stages on analysis which can be done sequentially and are independent. Analyses can be interrupted at the end of each stage after saving the output dataframe.
1. Step - Basic object detection This step creates a “_features.json” file which contains a table of objects detected in each frame. Beyond finding the center of mass of an object, no further image analysis is done here.
2. Step - Linking objects into trajectories This results in individual files “_trajectory.json” for each tracked animal.
3. Step - Analysing the details of object shapes This step is doing the heavy lifting: It extracts centerlines, widths, contours and other object descriptors from the objects
All subsequent analyses steps add ‘columns’ to the data, and thus features is a subset of trajectories is a subset of results.
1. Setup
def setup(parameterfile, inPath, outPath, movie):
"""helper function to simplify the setting up before analysis. Handles path checking and creating, parameter reads ad data reads."""
... # see the notebooks directory for more information
return logger, param, rawframes, lawn, outfile, imfile
Input parameters
parameterfile = r"C:\Users\bonnard\Documents\GitHub\PharaGlow\AnalysisParameters_1x.json"
inPath = r"C:\Users\bonnard\Documents\DATA\1_rawdata\demo_data"
outPath = r"C:\Users\bonnard\Documents\DATA\3_pharaglow\demo_data"
movie = "demo_data"
nWorkers = 4
depth = 'uint8'
save_minimal = True
lawnPath = None #"/opt/data/Lawns/"
logger, param, rawframes, lawn, outfile, imfile = setup(parameterfile, inPath, outPath, movie)
2. Object detection
Create binary masks
start = timeit.default_timer()
# detecting objects
logger.info('Binarizing images...')
masks = tracking.calculateMask(rawframes,
minSize = param['minSize'],
bgWindow = param['bgWindow'],
thresholdWindow = param['thresholdWindow'],
smooth = param['smooth'],
subtract = param['subtract'],
dilate = param['dilate'],
tfactor=param['tfactor'])
stop = timeit.default_timer()
logger.info(f"binary masks created ({stop - start}s)")
INFO:PharaGlow:Binarizing images...
INFO:PharaGlow:binary masks created (2.287s)
Make sure the thresholding worked otherwise change parameters
# Select a rawframe to visualize
t = 400
if t> (len(rawframes)-1):
# Check if the selected rawframe is present otherwise t=0
print(f"Warning ! Max {len(rawframes)} rawframes. {t} changed to 0")
t=0
print(f"rawframe {t} to visualize ")
rawframe 400 to visualize
Visualize the raw data
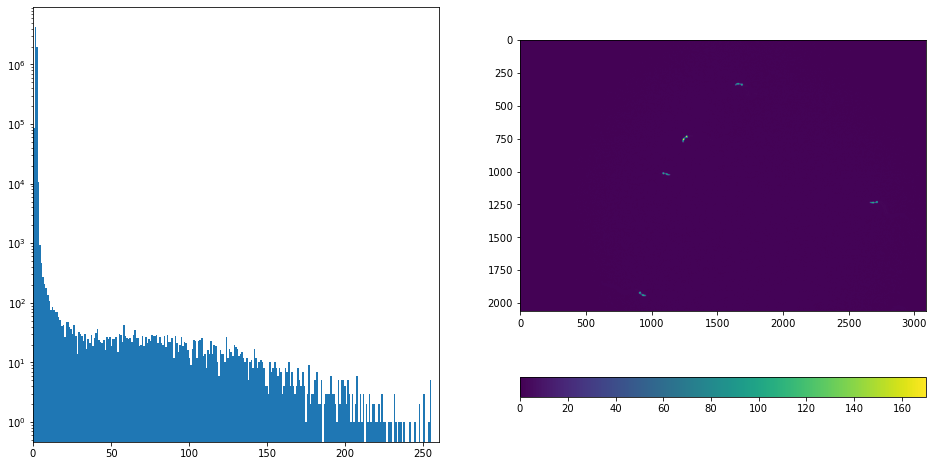
plt.figure(figsize=(16,8))
plt.subplot(121)
# Plot the histogram of the pixel intensity values of the rawframe
plt.hist(rawframes[t].ravel(), bins=256, log=True)
plt.xlim(0, 260) # xlim for 8 bits image
plt.subplot(122)
# Adjust the color limit for the rawframe for vizualisation only
color = (0,170) # 0<=color<=255 for 8 bits image
# color = None
plt.imshow(rawframes[t],clim = color)
plt.colorbar(orientation='horizontal');
plt.savefig(os.path.join(outPath,f'{date.today()}_{movie}_frame{t}_px_hist.pdf'))
Show the mask and detected objects
# %matplotlib qt
from skimage.measure import label, regionprops
plt.figure(figsize=(16,6))
plt.subplot(121)
# Show the rawframe
plt.imshow(rawframes[t],clim= color)#+lawn)
if lawn is not None:
# Show the lawn
plt.contour(binLawn, alpha=0.5, cmap='pink')
plt.subplot(122)
# Show the masks and their size [px]
plt.imshow(masks[t])#[:600,1000:])#[500:1500,2000:3500])#[:,2500:])
# print(np.min(masks[t]))
label_image, num = label(masks[t], background=0, connectivity = 1,return_num=True)
print(f"{num} detected objects")
for region in regionprops(label_image):
plt.text(region.centroid[1]+50, region.centroid[0], region.area, color ='w')
plt.tight_layout()
# save the pdf
plt.suptitle(f"Rawframe and masks (#{num}) rawframe {t} ({movie})", fontsize=14)
plt.savefig(os.path.join(outPath,f'{date.today()}_{movie}_frame{t}_masks.pdf'))
5 detected objects

Detecting individual objects and tracking or use multiprocessing to speed up feature detection
This section will go through all frames and find worm-sized (as specified by the parameters) objects. It creates a pd.Dataframe containing these and a stack of images (numpy array) that contain a cropped area around each worm. Note: Each worm image will be length x length x 8bit. So with 30 worms per image you expect the image array to be 6Gb/10 minutes.
start = timeit.default_timer()
logger.info('Detecting features...')
logger.info(f'...with {nWorkers} workers')
objects, images = util.parallel_analysis((masks, rawframes), param, tracking.parallelWorker, framenumbers = None, nWorkers = nWorkers, output= None, depth = depth)
# create a link between image and dataframe
objects['im_idx'] = np.arange(len(objects))
stop = timeit.default_timer()
logger.info(f"features detected ({stop - start}s)")
INFO:PharaGlow:Detecting features...
INFO:PharaGlow:...with 4 workers
Analyzing image 0 of 999
Analyzing image 100 of 999
Analyzing image 200 of 999
Analyzing image 300 of 999
Analyzing image 400 of 999
Analyzing image 500 of 999
Analyzing image 600 of 999
Analyzing image 700 of 999
Analyzing image 800 of 999
Analyzing image 900 of 999
INFO:PharaGlow:features detected (319.0189164s)
# Files monitoring
logger.info(f" Number of frames in features:{objects['frame'].nunique()}")
if len(rawframes) != len(objects['frame'].unique()):
logger.warning(f" Number of frames in features ({objects['frame'].nunique()}) and the number of rawframes ({len(rawframes)}) don't match !")
INFO:PharaGlow: Number of frames in features:999
Visualize results of object detection
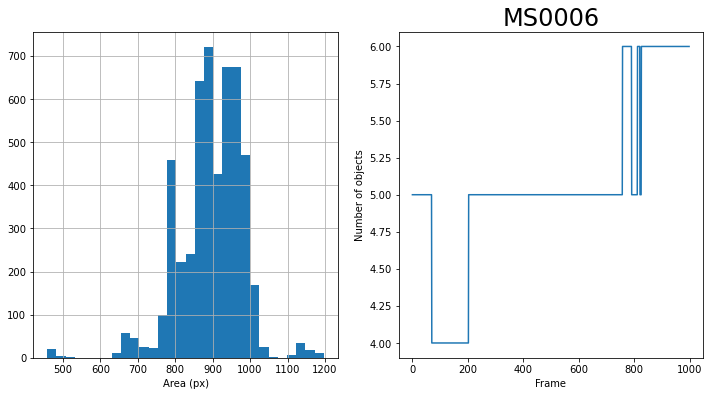
### Show the area of all objects
plt.figure(figsize=(12,6))
plt.subplot(121)
objects['area'].hist(bins = 30)
plt.xlabel('Area (px)')
plt.subplot(122)
objects['frame'].value_counts().sort_index().plot()
plt.ylabel('Number of objects')
plt.xlabel('Frame')
# save the pdf
plt.title(f"{movie}", fontsize=24)
plt.savefig(os.path.join(outPath,f'{date.today()}_{movie}_objects_.pdf'))
logger.info(f"features.area.min():{objects.area.min()}") # region.area > params['minSize']
logger.info(f"features.area.max():{objects.area.max()}") # region.area < params['maxSize']
INFO:PharaGlow:features.area.min():458
INFO:PharaGlow:features.area.max():1197
Save features and images
start = timeit.default_timer()
# saving features
logger.info("Saving features...")
objects.info(memory_usage='deep')
objects.to_json(outfile.format('features', 'all'), orient='split')
stop = timeit.default_timer()
logger.info(f"features saved as {outfile.format('features', 'all')} ({stop - start}s)")
start = timeit.default_timer()
# saving images
imsave(imfile.format('images', 'all'), images)
stop = timeit.default_timer()
logger.info(f"images saved as {imfile.format('images', 'all')} ({stop - start}s)")
This step results in a features file which contains center-of-mass tracking information, and an images files which are cropped regions of interest around a worm.
(Optional) Load features and images if continuing prior analysis
%%time
# leaving this here for re-analysis
if False:
# Load feature
start = timeit.default_timer()
logger.info("Loading features...")
objects = io.load(outfile.format('features', 'all'), orient='split')
images = pims.open(imfile.format('images', 'all'))
stop = timeit.default_timer()
logger.info(f"features loaded ({stop - start}s)")
Wall time: 0 ns
3. Creating trajectories
Link objects to trajectories using trackpy and interpolate short misses
logger.info('Linking trajectories...')
logger.info(f"Parameter searchRange: {param['searchRange']} px")
logger.info(f"Parameter memory: {param['memory']} frames")
INFO:PharaGlow:Linking trajectories...
INFO:PharaGlow:Parameter searchRange: 10 px
INFO:PharaGlow:Parameter memory: 30 frames
trajectories = tp.link_df(objects,param['searchRange'], memory = param['memory'])
logger.info(f"Number of trajectories after linking: {len(trajectories['particle'].unique())}")
INFO:PharaGlow:Number of trajectories after linking: 8
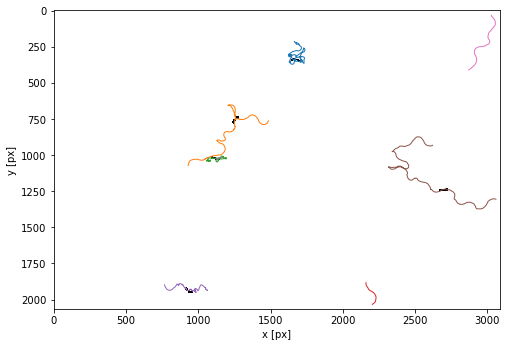
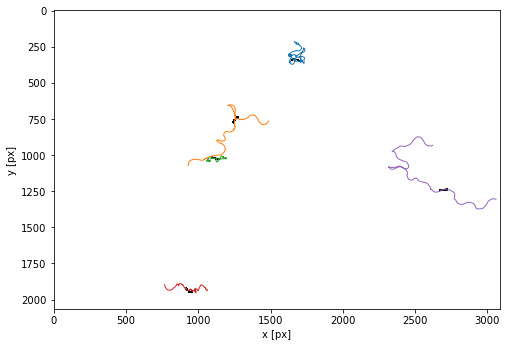
Show the trajectories
plt.figure(figsize=(8,8))
tp.plot_traj(trajectories, colorby = 'particle', superimpose=1-masks[t],label=False);
logger.info(f"Filtering out trajectories which last less than the minimal duration ({param['minimalDuration']} frames)...")
logger.info(f"Nb of trajectories before filtering: {trajectories['particle'].nunique()}")
trajectories = tp.filter_stubs(trajectories,param['minimalDuration'])
logger.info(f"Nb of trajectories after filtering: {trajectories['particle'].nunique()}")
INFO:PharaGlow:Filtering out trajectories which last less than the minimal duration (600 frames)...
INFO:PharaGlow:Nb of trajectories before filtering: 8
INFO:PharaGlow:Nb of trajectories after filtering: 5
fig = plt.figure(figsize=(8,8))
ax = tp.plot_traj(trajectories, colorby = 'particle', superimpose=1-masks[t],label=False);
# save the pdf
ax.set_title(f"{movie}", fontsize=24)
fig.savefig(os.path.join(outPath,f'{date.today()}_{movie}_trajectories_filtered.pdf'))
# with labels
fig = plt.figure(figsize=(8,8))
ax = tp.plot_traj(trajectories, colorby = 'particle', superimpose=1-masks[t],label=True);
# save the pdf
ax.set_title(f"{movie}", fontsize=24)
fig.savefig(os.path.join(outPath,f'{date.today()}_{movie}_trajectories_filtered_labelled.pdf'))
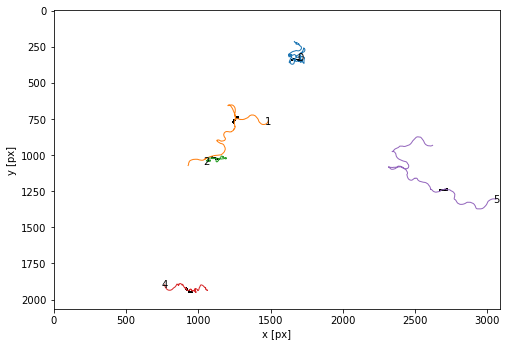
Save individual trajectories & add the missing images to interpolated trajectories
Here we do multiple things: Add missing rows to the trajectory, create a separate image stack for each animal and save the trajectories.
logger.info(f"Saving {trajectories['particle'].nunique()} trajectories to separate files...")
start = timeit.default_timer()
for particle_index in trajectories['particle'].unique():
tmp = trajectories[trajectories.loc[:,'particle'] == particle_index].copy()
ims = images[tmp['im_idx']]
ims = np.array(ims, dtype = 'uint8')
# generate an interpolated trajectory where all frames are accounted for
traj_interp, ims_interp = tracking.interpolate_helper(rawframes, ims, tmp, param)
# save the new single worm movie
imsave(imfile.format('images', particle_index), np.array(ims_interp, dtype='uint8'))
# save the trajectory
traj_interp.to_json(outfile.format('trajectories', int(particle_index)), orient='split')
stop = timeit.default_timer()
logger.info(f"trajectories saved as json files ({stop - start}s)")
INFO:PharaGlow:Saving 5 trajectories to separate files...
INFO:PharaGlow:trajectories saved as json files (0.692s)
3. Run the whole pharaglow feature extraction
start = timeit.default_timer()
# save only minimal outputs - reduces save by approx factor 3
# analyze all trajectories
for fn in os.listdir(outPath):
file = os.path.join(outPath,fn)
if os.path.isfile(file) and f'{movie}_trajectories_' in fn and fn.endswith('.json'):
particle_index = int(fn.split('.')[0].split('_')[-1])
traj = io.load(file, orient='split')
# load images
images = pims.open(imfile.format('images', particle_index))
if len(traj.index)<1:
print('Skipped', file)
continue
logger.info('Analyzing trajectory:%s', fn)
tmp,_ = util.parallel_analysis((images,), param,\
parallelWorker= run.parallel_pharaglow_run, framenumbers = traj['frame'], nWorkers = nWorkers, output= None)
# add basic image properties
tmp['Imax'] = np.max(images, axis=(1,2))
tmp['Imean'] = np.mean(images, axis=(1,2))
tmp['Imedian']= np.median(images, axis=(1,2))
tmp['Istd']= np.std(images, axis=(1,2))
tmp['Area2'] = [np.sum(mask) for mask in tmp['Mask']]
# remove some columns to make the result smaller
if save_minimal:
tmp = tmp.drop(['Mask', 'SkeletonX', 'SkeletonY', 'ParX', 'ParY',
'Xstart', 'Xend', 'dCl', 'Widths', 'Contour', 'Gradient',
'Kymo', 'KymoGrad', 'Similarity', 'Xtmp'], axis = 1, errors = 'ignore')
# add the basic tracker info - you can also keep these as separate files
tmp = tmp.merge(traj, on='frame', how = 'outer')
# drop nans to allow post processing
tmp = tmp.dropna()
print(tmp.info())
# run some stuff on the whole dataframe.
run.pharynxorientation(tmp)
# extract pumps
tmp[['pumps']] = tmp.apply(\
lambda row: pd.Series(features.extractPump(row['Straightened'])), axis=1)
# get more exact entry location
if lawn is not None:
tmp['insideHead'] = tmp.apply(\
lambda row: pd.Series(features.headLocationLawn(row['Centerline'],row['slice'], binLawn)), axis=1)
tmp['insideHeadIntensity'] = tmp.apply(\
lambda row: pd.Series(features.headLocationLawn(row['Centerline'],row['slice'], lawn)), axis=1)
tmp.to_json(outfile.format('results', particle_index), orient='split')
if save_minimal:
logger.info('minimal information saved')
stop = timeit.default_timer()
logger.info(f"Whole pharaglow features extracted ({stop - start}s)")
INFO:PharaGlow:minimal information saved
INFO:PharaGlow:Whole pharaglow features extracted (963.5043299000099s)
Run PharaGlow on your data
Raw files requirement
Raw data are tiff files typically obtained from simultaneously recording of up to 50 adults worms at 30 frames per second at 1x magnification. Typical use is by interacting through the notebook which contains the whole pipeline from raw movies to final data.
Parameters file
PharaGlow requires a json parameter file with the parameters that are editable by you. A default file comes with the repository, you can use it as a starting point (AnalysisParameters_1x.json). These parameters are:
Field |
Description |
|---|---|
subtract |
(0 or 1) Subtract the background from the movie for detection. Helps particularly with the higher resolution movies. |
smooth |
(integer, >= 0, in pixel) Should the image be smoothed. This helps to avoid breaking up the pharynx into two parts. |
dilate |
(integer, >=1) Binary dilation of the image. Can help to connect the worm if its broken up into two pieces. |
tfactor |
(float,[0-1]) Use rarely. If you have disparate sizes the automated threshold doesn’t work well. This factor multiplies the threshold value for binarization. Eg. for an 8-bit image, if the threshold is 150 and tfactor is 0.5 the image would be thresholded at 150*0.5=75. |
thresholdWindow |
(in frames) To get a threshold for binarization, use every nth frame of the movie. |
bgWindow |
(in frames) Calculate a static background on every nth image of the movie. If this is too short, you get a memory error. It can be as large as 500 frames for a full 18000 frame movie. |
length |
(in pixel) This sets the size of the extracted images around the center of the worm. It should be at least as large as the largest expected worm length. |
watershed |
(in pixel) When two or more worms touch, how large is an individual approximately. |
minSize |
(in pixel) Remove all objects smaller than this. |
maxSize |
(in pixel) Remove all objects larger than this (but a caveat here is when we have worm collisions where we allow the resulting segmentation to be a bit bigger). |
searchrange |
(in pixel) Describes how much we expect a worm to move frame-to-frame when we link particles together during tracking. This can be a bit bigger to allow for loosing the worm for a bit, but then you might get large perceived jumps in velocity. |
memory |
(in frames) When we loose a worm for a few frames, how long can gaps be until we call it a ‘new’ worm. |
minimalDuration |
(in frames) Filters out worm trajectories that are shorter than this. |
widthStraight |
(in pixel) How wide is a worm for the straightened image. |
pad |
(in pixel) crops a boundary around a worm for image analysis. this helps when the mask is a bit too small. |
nPts |
(integer) How many points along the centerline are we measuring. This should relate to the typical length of a worm. |
linewidth |
(integer) number of points to sample for a kymograph. 1 means only pixels directly on the centerline are taken. |
Run PharaGlow on a single data set
If you want to analyze one data set, run the notebook PharaGlowMain (PharaGlow > notebooks > PharaGlowMain.ipynb) as shown in the section above #Run PharaGlow on a demo dataset
Run PharaGlow on multiple data sets
If you want to analyze multiple data sets, you need (in PharaGlow > notebooks) :
pglow_batch_config.json
runPGlow_HPC.py
PharaGlowHPC.ipynb
API
https://scholz-lab.github.io/PharaGlow/build/html/pharaglow.html
References
Tracking is based on the package trackPy (http://soft-matter.github.io/trackpy/v0.4.2/).
License
scholz-lab/PharaGlow is licensed under the GNU General Public License v3.0